
It is possible that in the future, new evidence may be found to change the nuclear model of the atom.

These conclusions led to the nuclear model of the atom that we use today. The mass relationships between elements and compounds in chemical reactions ultimately relate back to the characteristics of the atoms of which they are composed. Atoms are the fundamental building blocks of all matter.

This suggested that the majority of the gold foil must be empty space but there must be small positive charges to reflect the alpha particles.įrom this experiment Rutherford concluded that: He found that most alpha particles went straight through the foil but a small percentage were scattered in different directions. Then, in 1909, Ernest Rutherford conducted an experiment where he fired positively charged alpha particles at a thin gold foil. This lead to the development of the “plum pudding model” where electrons were thought to be distributed randomly in a positively charged sphere, like fruit in a plum pudding. This spherical modelwas the accepted scientific opinion until 1909, when JJ Thomson discovered the electron. This model suggests that atoms are tiny spheres that couldn’t be broken into smaller parts. Electron configuration practice worksheet answer key Electron configuration. (i) The positive charge was concentrated in a small space at the centre, which is the nucleus.In 1803, John Dalton came up with the spherical model of the atom. plEntropy pogil Use the gas laws and kinetic theory to relate the pressure. What did Rutherford conclude from the observations of the $\alpha -ray$ scattering experiment?Īns: Rutherford proposed the nuclear model of an atom as There is balancing of charges, total positive charge is equal to the total negative charge.Ħ. Regular tables are available for exams, but rule lists are not available, so you can use rules to remember. Give the main features of Thomson’s Model for an atom.Īn atom hosts a sphere, which is positively charged, and the electrons are present in it and spread all over. Atomic structure practice 1 worksheet answer key. If you are still struggling to remember all the definitions. He first proposed a firm scientific basis named Dalton’s atomic theory.Īns: According to Thomson’s experiment, e/m ratio for an electron is $1.76\times \times $ ?ĥ. These worksheets have students explore the nature of atoms and their structure. An elements atomic structure refers to the structure of its nucleus and.
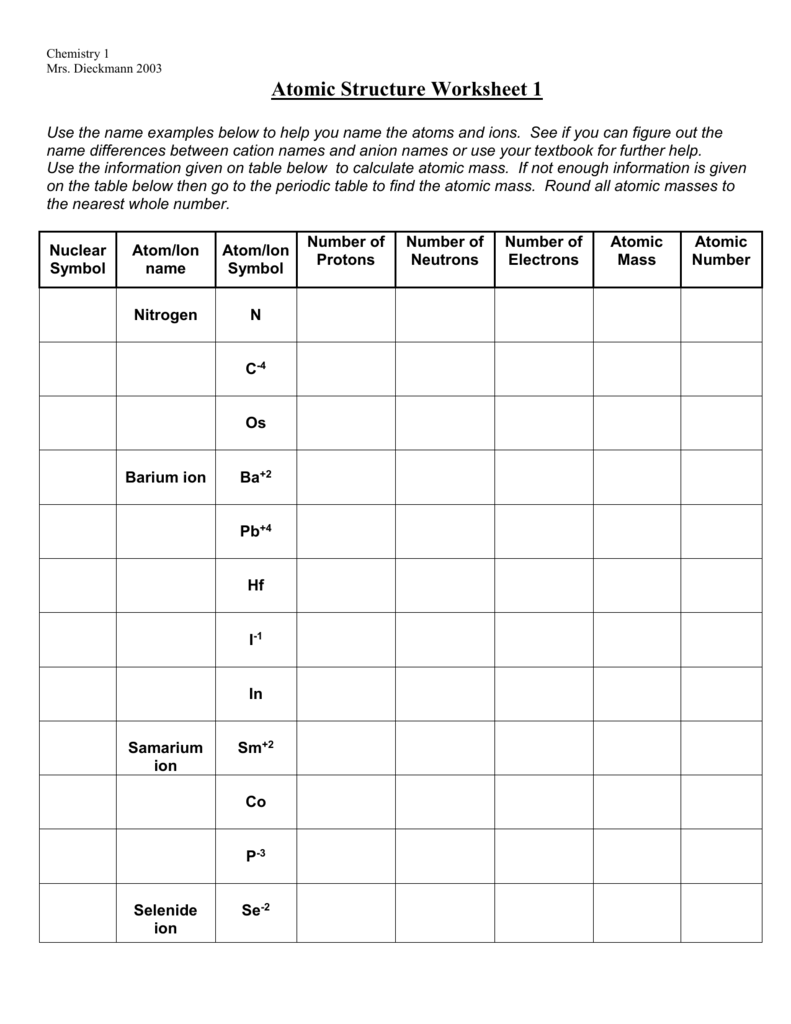
Name the scientist who first formulated the atomic structure.Īns: The atomic structure was formulated by John Dalton, a British teacher in 1808. Chemistry Worksheet Class 11 on Chapter 2 Structure of Atom with Answers - Set 1. Name the sub – atomic particles of an atom.Īns: The subatomic particles of an atom are:Ģ.


 0 kommentar(er)
0 kommentar(er)
